Secretomes in support of immune health
Immunis has developed a novel method to grow human cells for clinical use and direct them to become highly pure populations of defined cells. These cells secrete hundreds of factors that can benefit immune system development, modulation, and health. With age, everyone experiences a reduction in these valuable secretions. Our technology captures these powerful factors in our investigational secretome product (IMMUNA). IMMUNA is all-natural, all-human, and represents a perfectly balanced set of immune modulators that have the potential transform our health as we age.
IMMUNA Formulation
Immunis has developed a novel method to grow human cells for clinical use and direct them to become highly pure populations of defined cells. These cells secrete hundreds of factors that can benefit immune system development, modulation, and health. With age, everyone experiences a reduction in these valuable secretions. Our technology captures these powerful factors in our investigational secretome product (IMMUNA). IMMUNA is all-natural, all-human, and represents a perfectly balanced set of modulators at a physiological relevant concentration to potential transform our health as we age.

Pre-Clinical Safety and Efficacy Studies
Third-party preclinical safety studies confirmed the product to be safe and well tolerated.
Preclinical data supports that IMMUNA enhances metabolism and muscle regeneration. IMMUNA has also been shown to benefit vascular function, tissue inflammation, immune cell function, and exosome/extracellular vesicle composition in several age and immune-associated disease indications.
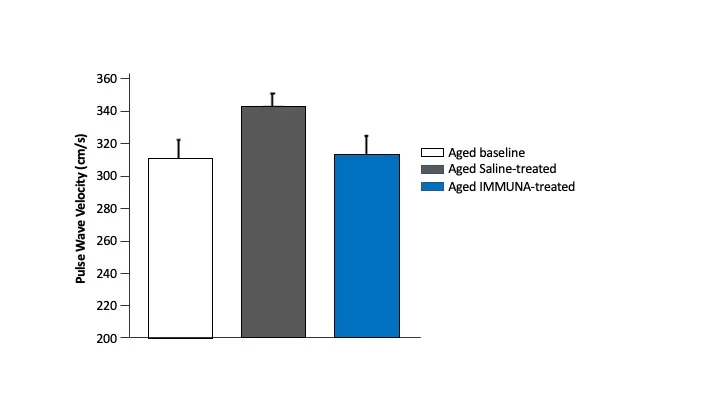
Pre-clinical studies of our investigational secretome product show a decrease in arterial stiffness with age.
Publications
Reversal of deficits in aged skeletal muscle during disuse and recovery in response to treatment with a secrotome product derived from partially differentiated human pluripotent stem cells
Dennis K Fix, Ziad S Mahmassani, Jonathan J Petrocelli, Naomi M M P de Hart, Patrick J Ferrara, Jessie S Painter, Gabriel Nistor, Thomas E Lane, Hans S Keirstead, Micah J Drummond