Phase 1/2a Clinical Trial for Muscle Atrophy
Clinical Trial Listing: An open-label dose escalation study to assess the safety and tolerability of IMMUNA in participants with muscle atrophy related to knee osteoarthritis
Target: Muscle atrophy; sarcopenia
Treatment: IMMUNA
ClinicalTrials.gov Identifier: NCT05211986
Every individual experiences a decline in immune system health as they age, which contributes to degenerative conditions throughout the body, including muscle atrophy. Age-related muscle atrophy (sarcopenia) and muscle wasting from disuse or disease significantly decreases quality of life. Mitigating the loss of muscle and improving muscle recovery remain unmet medical needs. Our goal is to utilize the secretome as a means of driving specific immune responses to overcome manifestations of age-related disease.
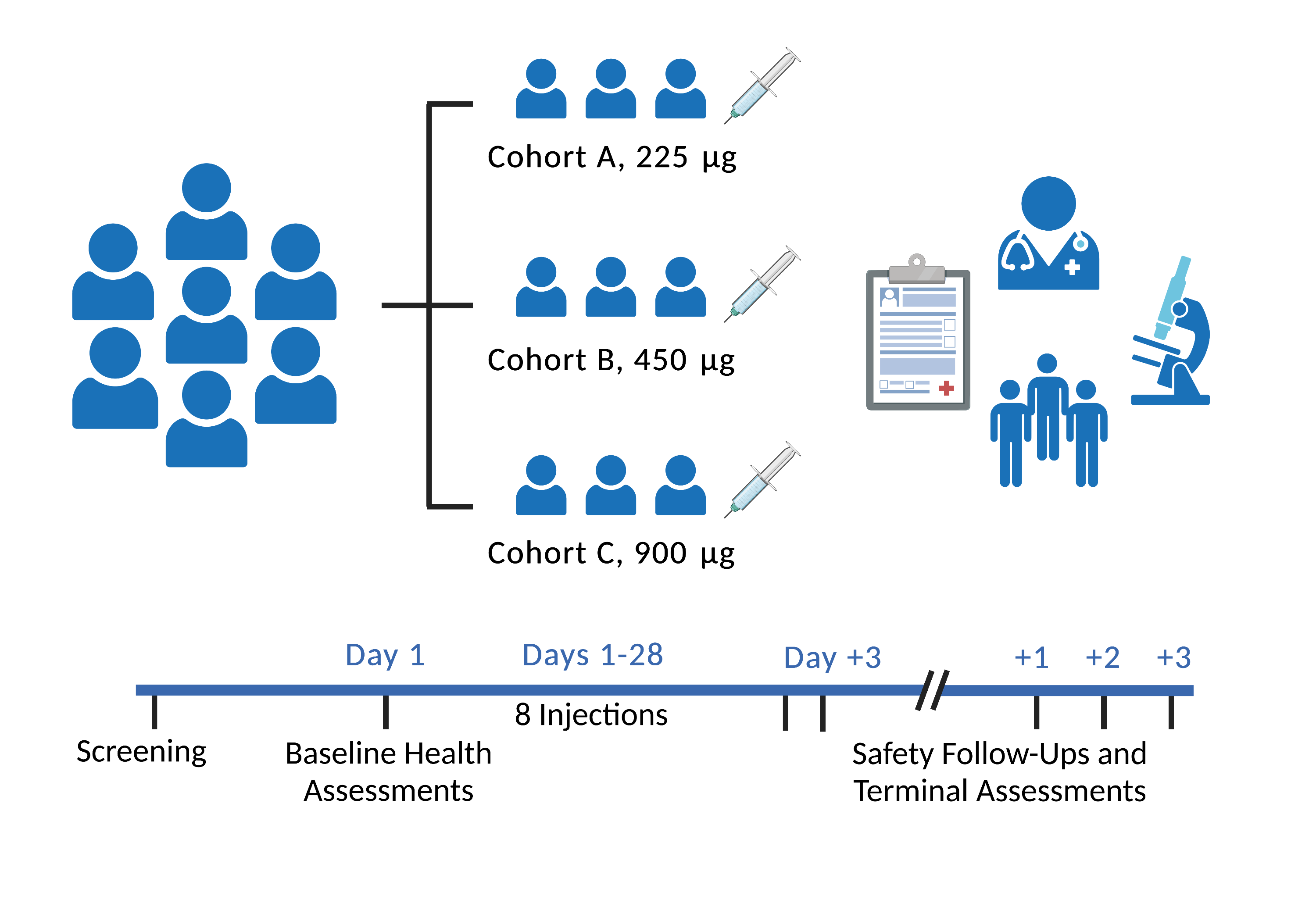
Research is being undertaken to determine whether IMMUNA can be beneficial in diseases characterized by muscle atrophy. IMMUNA is a secretome product derived from partially differentiated pluripotent cells concentrated with regenerative molecules. The FDA-approved Phase 1/2a clinical trial with IMMUNA is a single-center, open-label, dose-escalation study to assess safety and tolerability. IMMUNA is administered via intramuscular (im) injection twice per week for 4 weeks to participants with muscle atrophy related to knee osteoarthritis. A preliminary evaluation of the efficacy of IMMUNA in other indications such as Alzheimer’s Disease and multisystem senescence is also being performed.
The Phase 1/2a clinical trial is being conducted at the University of California Irvine Medical Center, UCI Health. UCI Health is part of the California Institute of Regenerative Medicine's Alpha Clinic Network. We invite you to learn more about these clinical trial opportunities.
Alpha Clinic at the UCI Medical Center




UCI Health is part of the California Institute of Regenerative Medicine’s (CIRM) Alpha Clinic Network, which is supporting Immunis’ Phase 1/2a clinical study. The investigations to assess the efficacy of Immunis’ investigational IMMUNA secretome product in the context of muscle atrophy is headed by Dean Wang, MD, alongside Brian Young Kim, MD, MS, Steven Yang, MD, MBA and Christopher Kroner, MD, MPH. The Alpha Clinics Network funded through CIRM has one unifying goal: to accelerate the development and delivery of cell therapies to patients.

Dean Wang, M.D.
Principal Investigator
Dr. Wang is an Associate Professor of Orthopaedic Surgery at UC Irvine School of Medicine. He is a board-certified orthopaedic surgeon who specializes in sports-related injuries of the knee, shoulder, hip, and elbow. His clinical expertise includes advanced, minimally invasive arthroscopic procedures and reconstructive joint surgery. He has specialized training in the field of biologic joint preservation procedures, including modern cartilage restoration techniques and arthroscopic hip preservation surgery. He is currently Chief of the Sports Medicine Division at UCI and a physician for UCI Athletics.

Brian Young Kim, M.D., M.S.
Sub-Investigator
Dr. Kim is an Associate Clinical Professor of Family Medicine at UC Irvine School of Medicine. He is a board-certified UCI Health specialist in family and sports medicine, providing multidisciplinary care for runners, youth, and female athletes. He serves as the Head Team Physician for the UCI athletics program and the Director of the Primary Care Sports Medicine fellowship.

Steven Yang, M.D., MBA
Sub-Investigator
Dr. Yang is an Assistant Clinical Professor of Orthopaedic Surgery at UC Irvine School of Medicine. He is a fellowship-trained orthopaedic surgeon specializing in adult reconstructive surgery with a focus on primary and revision hip and knee arthroplasty. He has specialized training in modern arthroplasty techniques, including anterior-approach hip replacement, computer-assisted joint replacement, patient-specific knee replacement, and partial knee replacements.

Christopher Kroner, M.D., MPH
Sub-Investigator
Dr. Kroner is an Associate Clinical Professor of Family Medicine at UC Irvine School of Medicine. He is board-certified in both sports medicine and family medicine. He practices both comprehensive primary care for patients of all ages and sports medicine specialty care. He is a team physician for the Anaheim Ducks and UCI Athletics and has several years of experience working with USA Water Polo, runDisney, and various community colleges and high schools.