Phase 2 Clinical Trial for Muscle Performance
Clinical Trial Listing: Safety and Efficacy of IMM01-STEM Against Placebo on Muscle Performance in Seniors With Obesity and Muscle Weakness
Target: Muscle performance; sarcopenic obesity
Treatment: IMM01-STEM or Placebo
Status: Enrolling
ClinicalTrials.gov Identifier: NCT06600581
Immunis is conducting a two-phase, placebo-controlled, dose expansion study with its multi active biologic, IMM01-STEM, in elderly sarcopenic obese individuals. The study will target safety parameters, body composition, and muscle strength and function. Additional metabolic, inflammatory, and muscle-adipose crosstalk markers will be monitored.
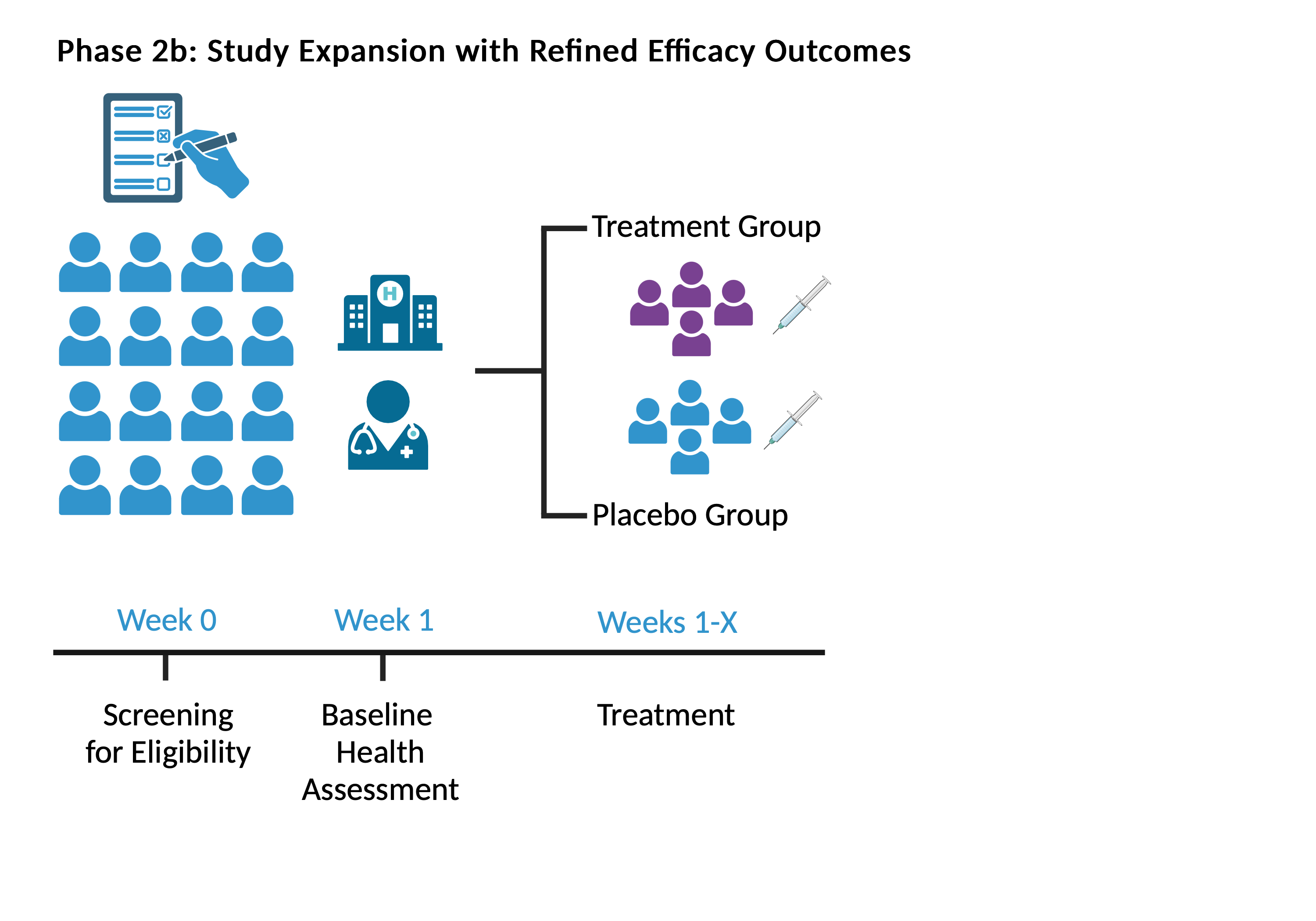
In the first phase of the trial, there will be four parallel dosing groups and one placebo control group. The treatment period will last one month and will be followed up with a safety assessment period. Our team will conduct interim analyses of the data from Phase 2a for design adaptation in Phase 2b.
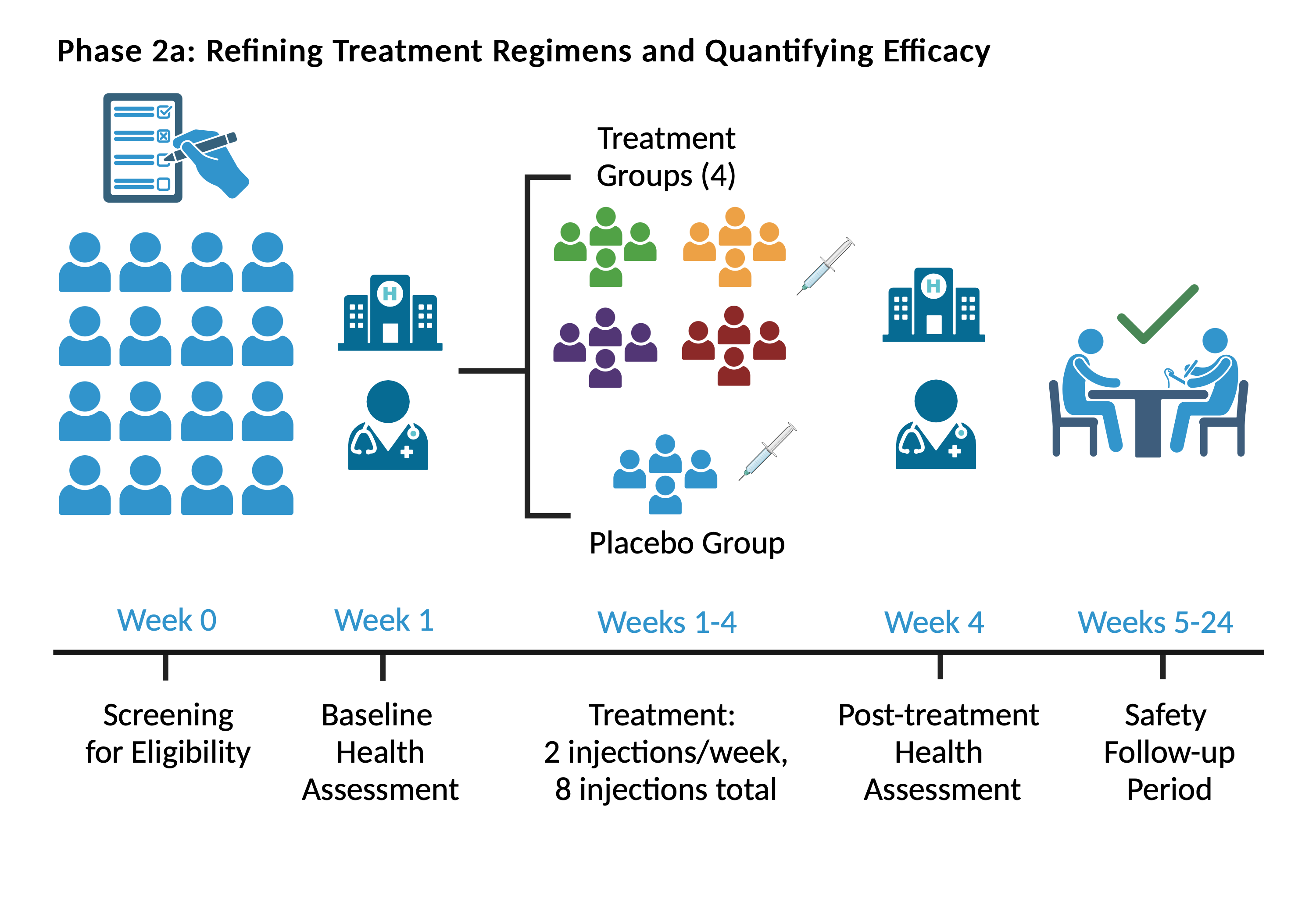
Treatments will be administered by intramuscular (IM) twice a week for four weeks. The dosing groups vary by IMM01-STEM total protein and will be adjusted for identical volumes or equivalent saline volume for placebo. Each participant will be evaluated at baseline, at the time of treatment administration, during the treatment period, then monthly until the end of trial.
At the interim point, the data from Phase 2a will be analyzed to identify the optimal treatment. The protocol will be adapted for Phase 2b to focus additional research on that most effective treatment arm. Post-adaptation, we will enroll new clinical trial participants in accordance with the new randomization rules in a 2-arm placebo-controlled study (Phase 2b).