Phase 1/2a Clinical Trial for Muscle Atrophy
Clinical Trial Listing: An open-label dose escalation study to assess the safety and tolerability of IMM01-STEM in participants with muscle atrophy related to knee osteoarthritis
Target: Muscle atrophy; sarcopenia
Treatment: IMM01-STEM
Status: Complete
ClinicalTrials.gov Identifier: NCT05211986
Every individual experiences a decline in immune system health as they age, which contributes to degenerative conditions throughout the body, including muscle atrophy. Age-related muscle atrophy (sarcopenia) and muscle wasting from disuse or disease significantly decreases quality of life. Mitigating the loss of muscle and improving muscle recovery remain unmet medical needs. Our goal is to utilize a multi-active biologic (IMM01-STEM) as a means of driving specific immune responses to overcome manifestations of age-related disease.
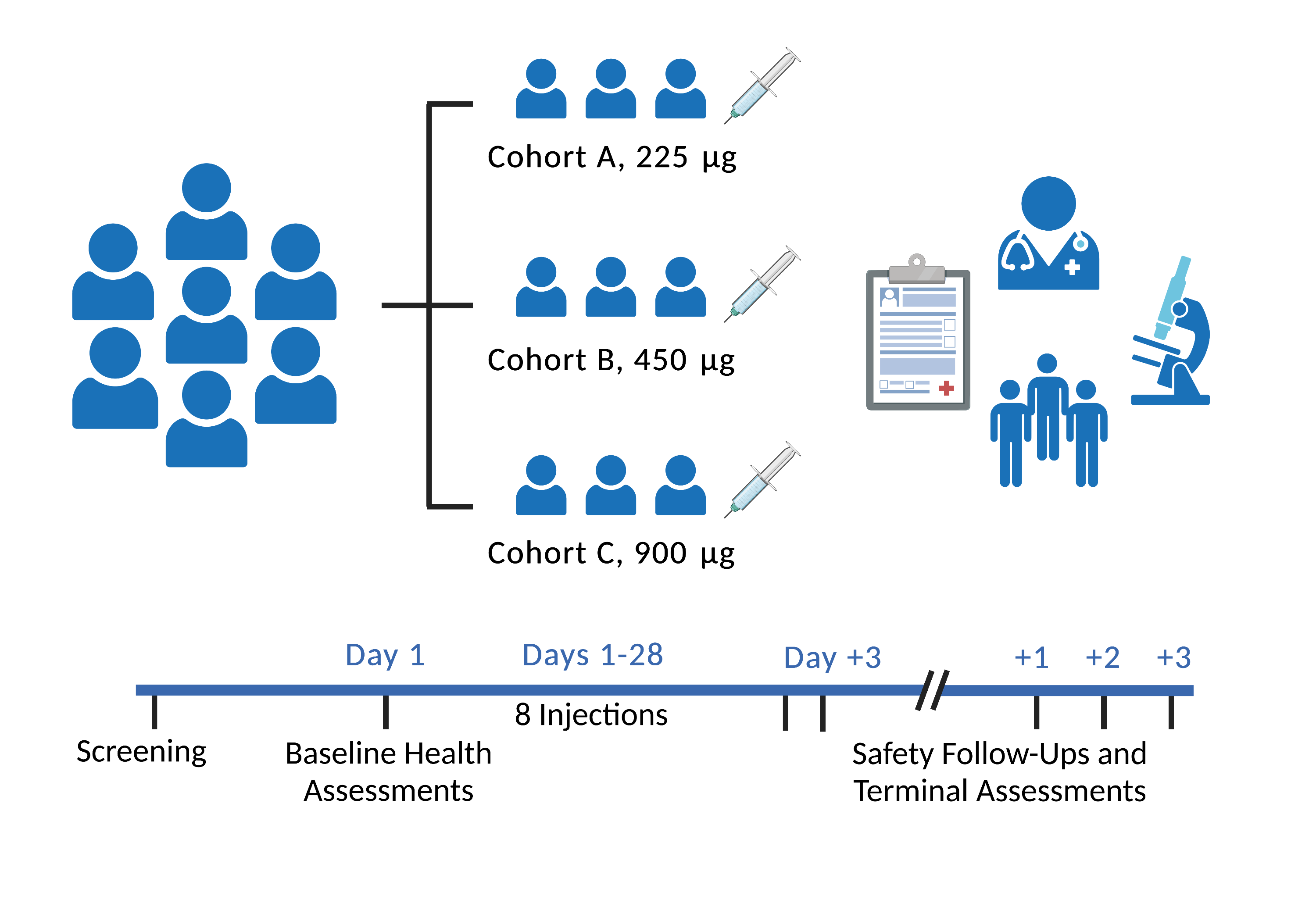
Immunis' STEM-MYO program is investigating whether its IMM01-STEM therapy can benefit individuals with diseases characterized by muscle atrophy. IMM01-STEM is a cell-free therapeutic derived from partially differentiated pluripotent stem cells and contains concentrated regenerative molecules. The FDA-approved Phase 1/2a clinical trial was a single-center, open-label, dose-escalation study to assess safety, tolerability and preliminary efficacy of IMM01-STEM in elderly participants with muscle atrophy related to knee osteoarthritis. IMM01-STEM was administered via intramuscular (im) injection twice per week for 4 weeks.
The Phase 1/2a was conducted at the University of California Irvine Medical Center, UCI Health. UCI Health is part of the California Institute of Regenerative Medicine's Alpha Clinic Network.